 Author
Author
|
Topic: Drug prices can't depend on who's buying (Read 3100 times) |
|
Walter Watts
Archon
    
Gender: 
Posts: 1571
Reputation: 8.25
Rate Walter Watts

Just when I thought I was out-they pull me back in
  
|
 |
Drug prices can't depend on who's buying
« on: 2009-11-19 02:28:26 » |
 |
Drug Topics
Nov 3, 2009
Drug prices can't depend on who's buying
By: Bruce T. Roberts, RPh
Imagine plunking down $20,000 for a new car and driving it off the lot, only to find your neighbor bought the same model for $2,000. And two doors down the street, someone else paid a mere $200 for the same vehicle. You'd probably have some choice words for the dealer's sales rep.
Welcome to the world of discriminatory pricing. In the prescription drug market, price discrepancies of this magnitude are common for independent community pharmacies and their patients. They cost consumers a fortune and largely determine winners and losers in the retail pharmacy community, rather than letting the marketplace decide. This fall, a Federal District Court in New York will consider a critical case: Drug Mart Pharmacy Corp. et al. v. American Home Products Corp. et al. It's an opportunity to address this issue and allow patients and health-plan sponsors to enjoy the benefits of lower drug prices and fair competition.
Some price volatility for goods and services is, of course, accepted and beneficial. And, certainly, bulk purchasers realize savings in economies of scale. But charging different prices to different purchasers to reduce competition or create a monopoly is wrong and anticompetitive. Congress outlawed it with the Robinson-Patman Act (1936 antitrust legislation), but for decades the practice has continued and become more common.
Drug manufacturers charge independent pharmacies one price for drugs; large chains and mail-order operations another; universities, hospitals and other entities another. A congressional subcommittee investigation found that pricing has became so variable that for $1, hospitals could purchase items that cost retail pharmacies $10 or even $100 to acquire. Some products acquired for a fraction of what they would cost the retail marketplace were even resold later by unscrupulous employees for a profit.
When independent community pharmacies pool some of their purchases to form buying groups, the economies of scale inexplicably vanish. The discounts afforded to comparably sized pharmacy or grocery chains remain out of reach.
The advent of the pharmacy benefit manager (PBM) has made this practice even more insidious. PBMs have further distorted the marketplace by demanding huge kickbacks from drugmakers to promote one drug over another to health-plan sponsors and patients. In light of this practice, it's not surprising that PBM mail-order businesses are charged less for drugs than retail pharmacies. Sadly, those savings don't make their way to the health plan or the patients, but instead go directly to the PBM CEOs.
The Robinson-Patman ban on discriminatory pricing was designed to be enforced by manufacturers who, presumably, would want to prevent anyone selling their product too far below prevailing market rates. However, those running pharmaceutical companies at the time decided to look the other way. Why? Two reasons.
First, to build brand prestige and market share with physicians and patients. After all, the brand used in hospitals is usually the brand patients are prescribed when they leave the hospital. Second, to curry favor with PBMs — the lucrative gatekeepers to patients. Have you ever been told the drug prescribed by the doctor is not the "preferred drug"? That's the giant PBMs at work, manipulating co-payments to promote a drug and squeeze more rebate dollars from its maker.
All this leads to an obvious conclusion: Retail consumers and independent community pharmacies are forced to subsidize discounts offered by manufacturers elsewhere in the pursuit of brand loyalty and market share. Manufacturers are caught in the vicious cycle of giving giant PBMs larger rebates in the hope that their drugs will gain preferred status. Meanwhile, PBMs use the rebates to help fuel their company profits. Discriminatory pricing means these medicines cost the average consumer and pharmacy far more than they should.
In the mid-1990s, thousands of pharmacies said, "Enough!" and filed a large class action suit citing violations of the Robinson-Patman Act. In 1996, they won a $723 million settlement.
But an important legal issue remains and will be considered in Federal District Court: Are these discriminatory pricing techniques illegal?
If the court agrees that they are, then nearly everyone wins. Manufacturers, freed from the burdens of PBM-imposed tiered pricing, could focus more resources on discovering the next breakthroughs and cures. Consumers and health-plan sponsors (employers, governments, unions, etc.) would benefit from lower prices and greater competition in the retail prescription-drug market. And community pharmacists could spend more time counseling patients about their medication needs and choices.
Bruce T. Roberts, RPh, is executive vice president and CEO of the National Community Pharmacists Association.
|
Walter Watts
Tulsa Network Solutions, Inc.
No one gets to see the Wizard! Not nobody! Not no how!
|
|
|
Hermit
Archon
    
Posts: 4289
Reputation: 8.50
Rate Hermit

Prime example of a practically perfect person
 
|
 |
Re:Drug prices can't depend on who's buying
« Reply #1 on: 2009-11-19 05:04:25 » |
 |
The problem with this article lies right here.[Bruce T. Roberts] All this leads to an obvious conclusion: Retail consumers and independent community pharmacies are forced to subsidize discounts offered by manufacturers elsewhere in the pursuit of brand loyalty and market share. Manufacturers are caught in the vicious cycle of giving giant PBMs larger rebates in the hope that their drugs will gain preferred status. Meanwhile, PBMs use the rebates to help fuel their company profits. Discriminatory pricing means these medicines cost the average consumer and pharmacy far more than they should. From the Re:Obama Administration Launches Deceptive Swine Flu Propaganda Blitz thread (reply 18) (as spotted by Letheomanic) we know that rather than subsidized discounts, what we are seeing is an invidious mark-up system, with hospitals sometimes (but not always) receiving goods at reasonable mark-ups, Walmart and Co at larcenous rates, pharmacies being stiffed and the consumer always being screwed.
With all the bowing, scraping and grovelling to the pharmaceutical industry currently engaged in by the administration to win their cooperation in helping to put together the ridiculous anti-health plan currently on the floor in Congress, I find it much more likely that, should the pharmaceutical industry lose in the Drug Mart Pharmacy Corp. et al. v. American Home Products Corp. et al case, that everyone will end up paying the small pharmacy price.
After all, it seems to be a dog eat dog kind of a country with the spoils to the victors and the population applauding, holding tea-parties celebrating Ayn Rand on lawns everywhere, even as their internal organs are displaced by the objects distending their colons.
|
With or without religion, you would have good people doing good things and evil people doing evil things. But for good people to do evil things, that takes religion. - Steven Weinberg, 1999
|
|
|
Walter Watts
Archon
    
Gender: 
Posts: 1571
Reputation: 8.25
Rate Walter Watts

Just when I thought I was out-they pull me back in
  
|
 |
Re:Drug prices can't depend on who's buying
« Reply #2 on: 2009-11-19 23:25:23 » |
 |
I just wanted to see how long it would take you to reach the same sad, inevitable conclusion that I reached.
Very speedy old friend. Very speedy.
I knew I could count on ya!
Best to you and yours.
Walter
Quote from: Hermit on 2009-11-19 05:04:25
The problem with this article lies right here.[Bruce T. Roberts] All this leads to an obvious conclusion: Retail consumers and independent community pharmacies are forced to subsidize discounts offered by manufacturers elsewhere in the pursuit of brand loyalty and market share. Manufacturers are caught in the vicious cycle of giving giant PBMs larger rebates in the hope that their drugs will gain preferred status. Meanwhile, PBMs use the rebates to help fuel their company profits. Discriminatory pricing means these medicines cost the average consumer and pharmacy far more than they should. From the Re:Obama Administration Launches Deceptive Swine Flu Propaganda Blitz thread (reply 18) (as spotted by Letheomanic) we know that rather than subsidized discounts, what we are seeing is an invidious mark-up system, with hospitals sometimes (but not always) receiving goods at reasonable mark-ups, Walmart and Co at larcenous rates, pharmacies being stiffed and the consumer always being screwed.
With all the bowing, scraping and grovelling to the pharmaceutical industry currently engaged in by the administration to win their cooperation in helping to put together the ridiculous anti-health plan currently on the floor in Congress, I find it much more likely that, should the pharmaceutical industry lose in the Drug Mart Pharmacy Corp. et al. v. American Home Products Corp. et al case, that everyone will end up paying the small pharmacy price.
After all, it seems to be a dog eat dog kind of a country with the spoils to the victors and the population applauding, holding tea-parties celebrating Ayn Rand on lawns everywhere, even as their internal organs are displaced by the objects distending their colons.

|
|
Walter Watts
Tulsa Network Solutions, Inc.
No one gets to see the Wizard! Not nobody! Not no how!
|
|
|
Hermit
Archon
    
Posts: 4289
Reputation: 8.50
Rate Hermit

Prime example of a practically perfect person
 
|
 |
Re:Drug prices can't depend on who's buying
« Reply #3 on: 2009-11-20 10:38:17 » |
 |
Grins. It doesn't take a pile driver to get through a wet paper bag. And when it is a bag of shit, using a pile driver is really messy.
And ours to you and yours
Love
Hermit&Co
|
With or without religion, you would have good people doing good things and evil people doing evil things. But for good people to do evil things, that takes religion. - Steven Weinberg, 1999
|
|
|
Fritz
Adept
    
Gender: 
Posts: 1746
Reputation: 7.93
Rate Fritz

  
|
 |
Re:Drug prices can't depend on who's buying
« Reply #4 on: 2009-11-20 18:08:44 » |
 |
In a related story ... though you'd think better yields to be had in the continental US then in Peru
Cheers
Fritz
'Fat for cosmetics' murder suspects arrested in Peru
Source: BBC News
Author:
Date: 2009-11-20
Four people have been arrested in Peru on suspicion of killing dozens of people in order to sell their fat and tissue for cosmetic uses in Europe.

The gang allegedly targeted people on remote roads, luring them with fake job offers before killing them and extracting their fat.
The liquidised product fetched $15,000 (£9,000) a litre and police suspect it was sold on to companies in Europe.
At least five other suspects, including two Italian nationals, remain at large.
Police said the gang could be behind the disappearances of up to 60 people in Peru's Huanuco and Pasco regions.
One of those arrested told police the ringleader had been killing people for their fat for more than three decades.
The gang has been referred to as the Pishtacos, after an ancient Peruvian legend of killers who attack people on lonely roads and murder them for their fat.
Human tissue
At a news conference in the capital, police showed reporters two bottles containing human body fat and images of one of the alleged victims.
One of the alleged killings is reported to have taken place in mid-September, with the person's body tissue removed for sale.
Cmdr Angel Toledo told Reuters news agency some of the suspects had "declared and stated how they murdered people with the aim being to extract their fat in rudimentary labs and sell it".
Police said they suspect the fat was sold to cosmetics and pharmaceutical companies in Europe, but have not confirmed any such connection.
Advertisement
The group allegedly sold body fat to be used in cosmetics in Europe
Human fat is used in modern cosmetic procedures but in most cases it is the patient's own fat that is used and under strict legal guidelines.
Medical authorities have expressed scepticism about a black market for human fat, partly because of the wide availability of fat for use in surgical procedures.
'Detailed confession'
Gen Felix Burga, head of Peru's police criminal division, said there were indications that "an international network trafficking human fat" was operating from Peru.
The first person was arrested earlier this month in a bus station in Lima, carrying a shipment of the fat.
The Associated Press news agency quoted Col Jorge Mejia as saying one of the suspects had described to police in detail how the victims were killed and their fat removed.
The suspect said the fat was then sold to intermediaries in Lima and that the gang's leader, Hilario Cudena, had been carrying out such murders for decades, AP reported.
The alleged buyers of the fat are also being hunted by police.
Source: The Star
Author:
Date: 2009-11.20
LIMA - Police in Peru infiltrated and cracked a gang that was kidnapping locals, killing them and draining their fat for use in the beauty industry. The gang has reportedly been active for 30 years in the remote areas of the Andes.
Three suspects confessed to killing five people, but the gang may have been involved in dozens more, said Col. Jorge Mejia, chief of Peru's anti-kidnapping police. He said one suspect claimed the gang wasn't the only one doing such killings.
Mejia said two of the suspects were arrested carrying bottles of liquid human fat and told police it was worth $15,000 (U.S.) a litre. The fat was sold to intermediaries in Peru's capital, Lima, and police suspect it was then sold to cosmetic companies in Europe, Mejia said Thursday, but he could not confirm any sales. The fat was reportedly to be used as an ingredient in anti-wrinkle cream.
Experts were quick to cast doubt on the scientific merit of the macabre scheme.
"In theory, yes, any lipid (fat) can be used in the production of cosmetics, but the quality would be, shall we say, variable. This is, of course, putting aside the ghoulish nature of this story, and the legal concerns, which preclude it happening," said Chis Flower, director general of the Cosmetic Toiletry and Perfumery Association in England.
A dermatology professor at Yale University, Dr. Lisa Donofrio, speculated that a small market may exist for "human fat extracts" to keep skin supple, but she said that scientifically such treatments are "pure baloney."
At a news conference, Peruvian police showed reporters two bottles of fat recovered from the suspects and a photo of the rotting head of a 27-year-old male victim. Suspect Elmer Segundo Castillejos, 29, led police to the head, recovered in a coca-growing valley last month, Mejia said.
Mejia said Castillejos recounted how the gang cut off its victims' heads, arms and legs, removed the organs, then suspended the torsos from hooks above candles that warmed the flesh as fat dripped into tubs below.
Six members of the gang remain at large, Mejia said. Among them was the band's alleged leader, Hilario Cudena, 56, who Castillejos told police has been killing people to extract human fat for more than three decades.
This year alone, at least 60 people are listed as missing in Huanuco province, where the gang allegedly operated, though the province is also home to drug-trafficking leftist rebels.
Mejia said police received a tip four months ago that human fat from the jungle was being sold in Lima. In August, he said, police infiltrated the band and later obtained some of the amber fluid, which a police lab confirmed as human fat.
On Nov. 3, police arrested Serapio Marcos Veramendi and Enedina Estela in a Lima bus station with a litre of human fat in a soda bottle. Their testimony led to the arrest of Castillejos three days later at the same bus station.
The three are charged with homicide, criminal conspiracy, illegal firearms possession and drug trafficking, according to a statement from Lima Superior Court. Police said they were searching for the alleged buyer.
Police dubbed the gang the "Pishtacos" after a Peruvian myth dating to pre-Columbian times of men who killed to extract human fat, quartering their victims with machetes.
Medical authorities contacted by The Associated Press said human fat is used in anti-wrinkle treatments – but is always extracted from the patient who is being treated, usually from the stomach or buttocks.
"There would be a risk of immunological reaction that could lead to life-threatening consequences" if fat from someone else were used, said Dr. Neil Sadick, a professor of dermatology at Cornell Weill Medical College in New York.
"This is indeed a very disturbing story," Andrea Hopp, chair of the Ontario chapter of the Society of Cosmetic Chemists, said in an email. She also wrote that she'd never heard of any scientific study of "human derived raw materials" in Canadian cosmetics.
Dr. Adam Katz, a professor of plastic surgery at the University of Virginia medical school, was incredulous when told about the Peruvian ring.
"I can't see why there would be a black market for fat," he said. "It doesn't make any sense at all, because in most countries we can get fat so readily and in such amounts from people who are willing and ready to donate that I don't see why there would ever be a black market for fat, of all tissues.
|
Where there is the necessary technical skill to move mountains, there is no need for the faith that moves mountains -anon-
|
|
|
Fritz
Adept
    
Gender: 
Posts: 1746
Reputation: 7.93
Rate Fritz

  
|
 |
Re:Drug prices can't depend on who's buying
« Reply #5 on: 2009-11-20 18:23:22 » |
 |
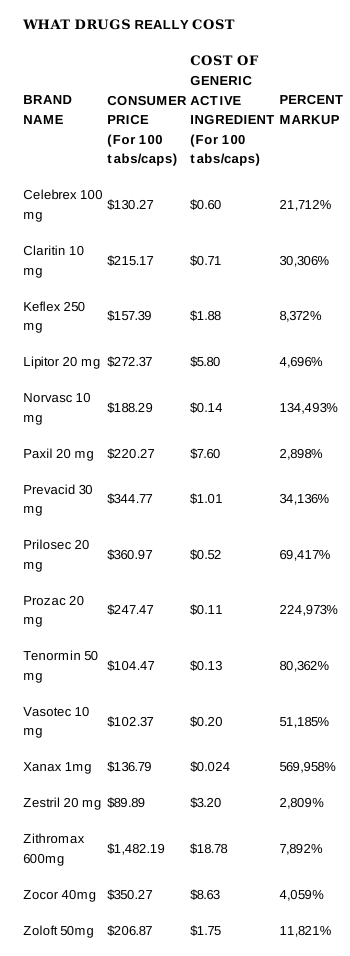
I have spoken with GPs that confirm this with their patients with blood pressure problems that were under control and when their HMO put them on generics, problems started and cleared again when they went back on the name brands. I am told that there is a 25% leeway on active ingredients permitted with generics in Canada but have been unable to confirm that.
So what I get from Hermit & Co, and Walter is we are getting royally put out no matter how these tablets crumble.
Are their any options or recourse .... you'd think the health insurance industry would be at least start poking away at the drug companies ... unless the drug companies are already paying the Health Insurances off ... hmmm
Cheers
Fritz
Source: The Road Back Foundation
Author:
Date:
Are Generic Drugs as Effective as Brand Name? - Not Always!
A number of patients with a history of good results on brand name antibiotics began experiencing difficulties when a generic was substituted. Therefore, if you have prescribed a brand name tetracycline for a patient using antibiotic therapy and have not specified d.a.w. or no substitutions, your patient is probably taking a generic version and may be having a less than significant response to the treatment. Some generic versions have been found to be ineffective for this treatment.
In order to market drugs, U.S. generic manufacturers must have a permit and approval from the Food and Drug Administration (FDA) indicating that the active ingredient is approximately the same as that of the brand name. The determination of drug approval is made according to whether it is pharmaceutically equivalent, bio-available, and bioequivalent.
Pharmaceutically Equivalent
Two drugs are considered pharmaceutical equivalents when they contain the same chemically active ingredient(s) and are identical in dosage form and strength. Tetracyclines such as minocycline are complex with many properties that may play an important part in treatment response in the arthritic patient. The fact that patients in remission (sometimes for years) while on antibiotic therapy saw a gradual return of symptoms when switched to a generic alerted us to a potential problem with some generics. In three test patients, these symptoms began to reverse immediately upon a return to the brand name version of the drug.
Pharmaceutical equivalence may be affected by many things.
1) variations in inert ingredients
2) plants in different parts of the world
may produce ingredients that vary in quality, and by batch and manufacturing methods. Until recently, 80% of drug ingredients came from plants in Western Europe. According to a NY Times article April 11, 1996, that is changing. Many ingredients are now being used from plants in China, Japan, South Korea, India and Eastern Europe where they are produced more cheaply. Bob Milanese, president of the National Association of Pharmaceutical Manufacturers, indicates that only a handful of these plants meet FDA standards. "Some others are questionable" due to the difficulty in finding people and budget to "get over and inspect these plants." Another factor which affects generic quality cited by the same article is the international buy outs and diversification allowing the combination of questionable ingredients into generic production.
3) In oral drugs, capsule content may be 7% over or 7% under the stated content, e.g. a 100 mg. capsule may be as low as 93 mg. or as high as 107 mg.
4) Manufacturers may shift their source of supply.
5) Once a drug has been approved by the FDA, manufacturers sometimes make changes to the formula which was originally submitted.
6) Many arthritic patients are elderly. The age of the patient may be a factor in pharmacokinetics. Digestive tract absorption of an oral drug may be altered by a variety of factors, including higher gastric pH, accelerated gastric emptying, and thinning and reduction of the absorptive surface. Bioavailability may be influenced by the increase (or decrease) in percent of body fat which is common in some with age. It may be even higher in sedentary persons (or persons in pain who are inactive in order to minimize discomfort.) This increased fat to lean ratio results in a reservoir for lipid-soluble drugs which is larger allowing those drugs to stay in the body longer, increasing the possibility of drug sensitivity by prolonging the half-life. Conversely, total water content declines with age. This decline allows a decreased volume of distribution for water-soluble drugs.
In addition to general approval, the FDA rates drugs with codes. All drugs with an "A" code are rated as being therapeutically equivalent; "B" coded drugs are those not rated equivalent Some pharmacies fill with B-rated drugs. At this time, it is recommended that no patient use a version of a drug with a B-rating. Clinical differences or serious bioequivalence problems with B-rated products have been reported for drugs such as prednisone, estrogen tablets, levodopa and phenytoin. In addition to The Orange Book, The Physician's Generix lists available generics as therapeutically equivalent or non-equivalent. Because the antibiotic protocol uses such low doses, leeway between versions which are effective and those which are not may be much more critical.
Bioavailability
In bioavailability, it can be assumed that the drug's effectiveness is related to the amount of product absorbed and the speed of absorption. However, in some cases, the pharmaceutically equivalent products can have different bioavailability. They may be absorbed either faster or slower than the brand name drug which may or may not be clinically significant.
The pH-dissolution profile of a product may have clinical relevance. Even if the coating is adequate to prevent release of the enzymes in the stomach where the ingredients are irreversibly inactivated, it may not dissolve at the pH of the duodenum after meals.
Bioequivalence
In bioequivalence studies, the goal of testing is to determine if the drugs are functionally equivalent. The FDA requires that any approved drug be effective within a 20% range of the original patented or brand name drug. This means that the effectiveness may be 20% greater or 20% less effective than the brand name so that two generic drugs could contain as much as a 40% difference from each other. Therefore, a drug may be legally chemically equivalent but not at the same time clinically equivalent. A study run on a generic of the anti-seizure, Tegretol, found the generic allowed breakthrough seizures.
An example of how the above factors may affect the bioavailability and clinical effectiveness is seen by applying these factors to tetracycline. At one extreme, a 500 mg. dose of tetracycline taken in 2-250 mg. capsules which is 20% lower in effectiveness, 7% low in the mg. amount in each capsule (14% dose total) and which is taken with food, decreasing the absorption rate (<50%), could provide as low as 136 mg of tetracycline that is available to the body. Correspondingly, the same 2-250 mg. capsules making a total dose of 500 mg. which is 20% more effective, 7% over on mg. in capsule and taken without food (increasing the absorption rate to 77%), provides 555 mg that is functionally available to the system. It should be noted the food-drug interaction is less a factor with minocycline and doxycycline as they are absorbed differently.
In addition to the ±20% difference allowed in bioavailability by the FDA and the ±7% of the stated capsule content allowed by the U.S. Pharmacopoeia, there are other considerations which should be considered when using a generic drug.
1) Some drugs lose potency while on the shelf, so drug companies increase the strength so as the drug ages, it will still provide a therapeutic level. This means patients who use the drug soon after production when the dose may be stronger may be getting an overdose.
2) There is a risk that a generic substitution could result in a change in serum concentration
3) Such a change may lead to signifi-cant adverse effects or loss of benefit
4) The risk that patients may receive different generics each time they fill their prescription, changing the response to the drug.
5) Cost of brand names is usually, but not always, higher than for a generic.
6) Blood tests can become necessary to determine adequate concentrations, excessive, possibly toxic concentrations or low, possibly ineffective concentra-tions
7) The cost of the time and effort spent in adjusting the dose (if needed)
Bioequivalence may be effected by the type of study; e.g. two brand name pharmaceutical equivalents were each compared with a placebo in separate trials but were not compared with each other for bioequivalence. Thus while each was effective, it cannot be assumed that they produce the same clinical effect. Bioequivalence studies are performed on healthy volunteers and thus may not account for the full pharmacologic and therapeutic impact of generic substitution on patients with disease.
Conclusion
A pharmacist may legally fill a prescription in the United States with either the brand name or a generic without consulting either the patient or the physician. A prescription may not even be filled consistently with the same generic. To assure continuity for the patient, the physician should indicate on the prescription no substitutions or dispense as written (daw).
The purpose of this article is not to condemn generic drugs for many are as effective as the brand name and even come from the same manufacturing company, but are repackaged and sold by another company as their own generic brand. Our purpose is, however, to provide a warning not to assume that all drugs with the same generic title are equal and will have the same clinical effect, even though many drug reps say they are equal. This is particularly true of the tetracycline family because it is one of the oldest families of antibiotics being first patented in 1953. Since a patent is good for 17 years, the original tetracycline has been available for generic reproduction for some 25 years.
References:
Brooke PA, Resistant Prices, A study of competitive strains in the antibiotic markets, 1976, Ballinger Pub.
Hendeles L, Hochhaus G, Kazerounian S, Generic and alternative brand-name pharmaceutical equivalents: Select with caution, Am J Hosp Pharm, 1993; 50:2, 323-329.
Medical Information Department, Lederle Labs, telephone conversations.
Mandell GL, Douglas RG, Jr., Bennett JE, Principals and Practice of Infectious Diseases, Wiley Medical Pub, 1985.
Mikati M, Bassett N, Schachter S, Double-blind randomized study comparing brand-name and generic phenytoin monotherapy, Epilepsia, 1992; 33:2, 359-364.
Oles KS, Penry JK, Smith LD, Anderson RL, Dean, JC, Riela AR, Therapeutic bioequivalency study of brand name versus generic carbamazepine, Neurology, 1992, 42:6, 1147-52.
Physician's Generix?, Data Pharmaceutica, 1996.
Reinstein PH, Regulatory status of pancreatic enzyme preparations, JAMA, 1990; 263:18, 2491-2492.
Stoughton RB, Are generic formulations equivalent to trade name topical glucocorticoids? Arch Derm, 1987; 123:9, 1312-1314.
Univ. of Chicago Drug Information, telephone conversation.
For insurance companies who will not cover brand name drugs when a generic is available, a blood test to determine concentration may be necessary for those using low dose antibiotics to provide data to require payment for the brand name drug.
To assure continuity for the patient, the physician should indicate on the prescription no substitutions or dispense as written (daw).
|
Where there is the necessary technical skill to move mountains, there is no need for the faith that moves mountains -anon-
|
|
|
Hermit
Archon
    
Posts: 4289
Reputation: 8.50
Rate Hermit

Prime example of a practically perfect person
 
|
 |
Re:Drug prices can't depend on who's buying
« Reply #6 on: 2009-11-20 23:15:04 » |
 |
re 'Fat for cosmetics'
The fat seems to be in the fire.
re Are their any options or recourse .... you'd think the health insurance industry would be at least start poking away at the drug companies ... unless the drug companies are already paying the Health Insurances off ... hmmm
US insurers and pharmaceutical companies tend to be inextricably interlocked in a very intricately woven web of shareholdings such that anything the one does ultimately benefits the shareholders of the other and vice-versa.
The genius is that they do not need to collude, pure naked greed is sufficient to ensure that all their interests are properly served (caviare and pink champagne for breakfast).
Love
Hermit&Co
|
With or without religion, you would have good people doing good things and evil people doing evil things. But for good people to do evil things, that takes religion. - Steven Weinberg, 1999
|
|
|
|




